In the ever-evolving world of clinical trials, streamlining processes and embracing digital transformation has become critical, even as it’s become more complicated. While many sites are pushing back adding new technology to an already overly-complex tech-stack, sponsors can still provide technology that improves a site’s workflows – it just needs to be done thoughtfully and methodologically.
Sandra Aguila Norbuena, a regional business systems lead at a top pharmaceutical organization, spoke at this year’s Research Revolution about how her organization was able to support and enable the sites they work with globally – in a process that proves that site adoption can be led, and supported by CRAs.
Understanding the challenges sites face
Sandra’s career in clinical research began as a study coordinator and later evolved into a Clinical Research Associate (CRA) and business systems lead, providing her with an in-depth understanding of the challenges faced by on-site teams. Her background gave her insight into the daily realities and document-heavy demands of trial sites and a unique perspective on why digital solutions like Florence SiteLink could be game-changers.
The catalyst for change: COVID-19
The COVID-19 pandemic catalyzed her organization’s shift to electronic systems. Travel restrictions and limited access to trial sites underscored the need for remote access to crucial documents. To keep critical studies from shutting down, Sandra’s organization initiated a company-wide journey to digitize site files, improve accessibility, and ensure business continuity using remote monitoring software. But with tens of thousands of sites around the world, with different workflows, different technology stacks, and different needs for support, getting them all to adopt not only a remote monitoring platform but also digital document exchange, and automated workflows seemed nearly impossible.
A structured rollout: from trials to global adoption
The adoption started gradually, focusing on initial trials to gather insights. This phased rollout, beginning in September 2021, collected feedback from Wave 0 and Wave 1 users. By the end of 2022, positive feedback paved the way for further adoption, ultimately leading to a larger-scale adoption plan. Structured workstreams focusing on communication, implementation, process refinement, and technical support were the key to scaling the adoption.
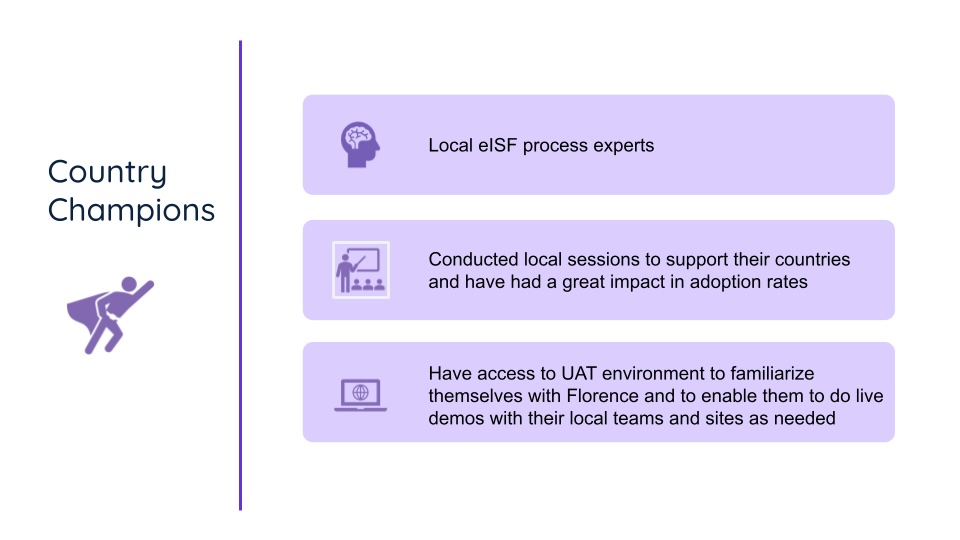
Country champions: localized support for global success
To address the unique requirements across regions, Sandra introduced “country champions.” These were something like local tech-support guides, who had in-depth, context-specific guidance on adopting the platform, addressing country-specific documentation needs, and navigating their local regulatory landscapes.
It proved to be a brilliant strategy. Sandra was able to train a smaller group of guides who then became the focal point for training their regions’ sites, making the rollout much smoother. The country champions then received continuous training and access to resources, to make sure they were able to fully facilitate localized adoption.
Building a culture of support and engagement
Site adoption at this scale required a culture shift. Global training sessions, weekly office hours, and a dedicated email support system in addition to the country champions, ensured that CRAs and trial site staff had continuous access to resources and could resolve issues quickly. Sandra’s organization also created job aids and role-specific materials to streamline user adoption and make it easy for team members to navigate the platform.
The results: worth the work
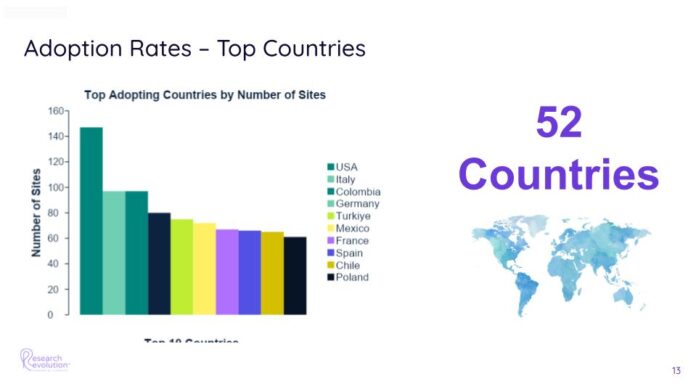
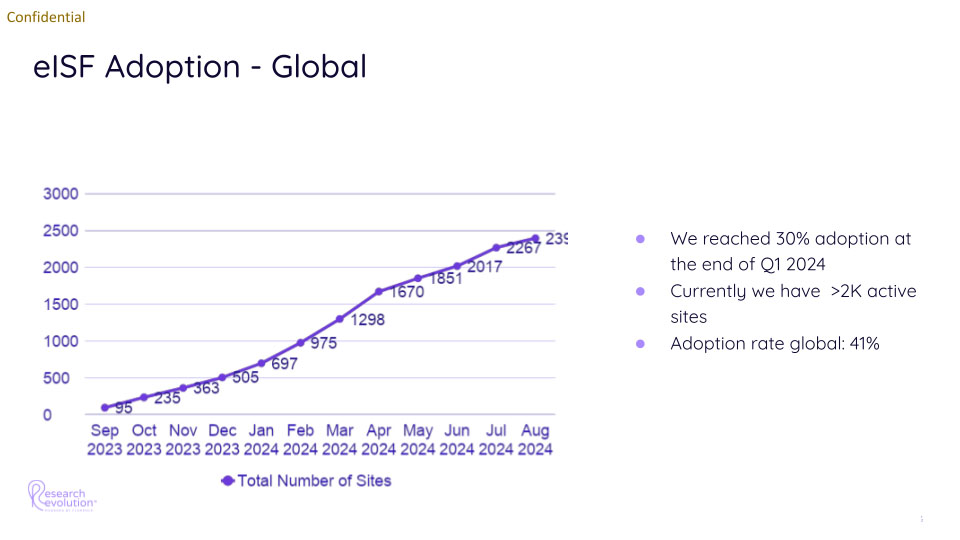
The results of this momentous effort were staggering: In just one year, the organization expanded from just 95 sites using the Florence platform to an incredible 2,400. By Q1 of the following year, adoption reached 41% of eligible sites, with ongoing efforts to increase global adoption rates.
Key Takeaways
For Sandra, there were five key elements that underpinned her organization’s success in the site adoption:
- Engagement: Repetitive messaging through diverse channels to ensure understanding and retention.
- Support and Involvement: High-level sponsorship and roadshows to communicate Florence’s value across various roles.
- Flexibility: Allowing sites to adapt Florence to their needs while maintaining a core structure.
- Localization: Country champions provided local expertise to align Florence with unique regional requirements.
- Continuous Improvement: Ongoing feedback collection to address evolving user needs and maintain satisfaction.
Looking Forward
Sandra’s story demonstrates how site adoption is not just a matter for sites to manage. Sponsor organizations can have a tremendous impact on the work their sites do when they work in concert with them. Change management, and by extension site adoption, are matters that sites and sponsors need to – and can – come together on.
You can watch Sandra’s Research Revolution session here, and let us know what you think!